Upon transport to the OR, the patient was receiving the above mentioned infusions, plus insulin 4 u/hr, diltiazem for new onset AF, Bicarbonate drip, and precedex at 0.2 mcg/kg/hr. These were all continued during surgery with the exception of the diltiazem.
Upon arrival to the OR I placed a left radial arterial catheter for invasive pressure monitoring. US was used and visualizing of the artery revealed a virtually pulseless distal artery despite NIBP blood pressures reading 100/50 mmHg. After cannulation, there was almost no pulistile flow from the catheter causing me to question whether I had cannulated the artery or a vein. After connection to the transducer, an obviously arterial tracing was visualized on the monitor. However, there was a very wide discrepancy between the NIBP reading and the AL reading. In general, the NIBP of the same arm was 45 mmHg higher for systolic pressures. All attempts to reduce this discrepancy failed. The waveform was not dampened, there was excellent blood flow when withdrawing from the AL, the transducer was carefully zeroed twice, and placed at a similar level as the NIBP cuff. The cuff was on the same arm as the AL. Therefore, throughout the case, the AL was used mostly for trending, while I chose to accept the NIBP reading as more indicative of the actual pressure experienced by the patient.
Here are some serial ABGs
The patients labs were as follows: (day of surgery was 2/16).
ABGs (serial)
2/13--7.2
2/14--7.37
2/15--7.5
2/15--7.3 19:00 (lactate 7.2)
2/16--7.013
2/17--7.027
Na+: 138 mEq/L
K+: 4.2 mEq/L
Cl-: 99 mEq/L
CO2: 21
BUN: 54 mg/dL
Cr: 4.3 mg/dL
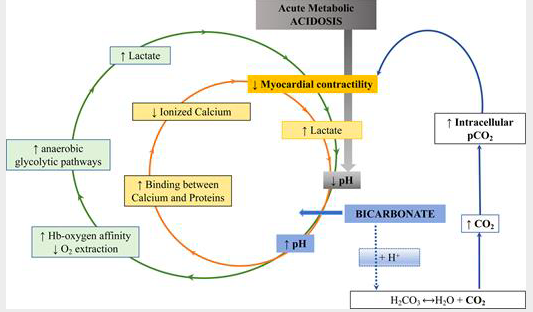
The patient was on a bicarb infusion and I continued this to the OR. I admit that I found this unusual and wondered what the evidence was to support treating severe lactic acidosis from severe ischemic colitis with bicarbonate therapy. Indeed, severe metabolic acidosis is feared by clinicians for good reason. Low pH (<7.2) predicts mortality in ICU. It is also associated with impaired cardiac contractility, systemic vasodilatation, pulmonary vasoconstriction, arrhythmias, altered oxygen delivery, altered renal blood flow, cerebral edema, diaphragm dysfunction, energy failure with decreased adenosine triphosphate production, and immune response impairment. However, the intuitive therapy of sodium bicarbonate given IV does not always prove therapeutic. Multiple studies in critically ill patients with metabolic acidosis have not found any benefit to bicarbonate therapy for improving outcomes. As an example, a robust study of 1700 patients was unable to find any benefit for those who received sodium bicarbonate for treatment of severe metabolic acidosis in the setting of sepsis [7]. This was replicated in two other large multi center RCTs where sodium bicarb did not prove beneficial [8]. Other studies have also not shown benefit. And, in a retrospective study, sodium bicarbonate infusion actually was an independent predictor of increased mortality [9]. However, in one small study, patients with lactic acidosis AND AKI did have improved mortality when treated with bicarb [1]. In studies on patients in code situations, bicarb therapy has not improved outcome and is likely contraindicated. Bicarb is most definitely indicated for patients with RTA I, or proximal RTA where bicarbonate is wasted, or not efficiently reabsorbed by the kidneys. However, RTA I leads to a chronic metabolic acidosis and is not relevant to our patients condition. To me, it is not clear that our patient met the criteria of the patients in the study that found a benefit of bicarb therapy. Prior to surgery the patient was anuric and had near complete renal failure, as opposed to AKI. In the above mentioned study, it should be noted that the authors found that in patients who did not have AKI and were treated with sodium bicarb, there was an increase in mortality after correction for disease severity. The authors noted that this may be related to the fact that severe acidosis inhibits the enzyme phosphofructokinase which is responsible for the production of lactate. By increasing pH, this enzyme is unleashed to continue to produce additional lactate worsening the acidemia unless the underlying cause of the production of lactate is corrected. It is of note, that in the cited above study, patients treated with sodium bicarb did not see a reduction of blood lactate levels to the degree of those patients with a similar acidemia who did not receive sodium bicarb therapy. It should be noted that in non mechanically ventilated patients, sodium bicarb therapy for metabolic acidosis can be particularly detrimental since this therapy rapidly increases gaseous CO2 in the blood. As PaCO2 increases, a respiratory acidosis may ensue, and as CO2 rapidly enters cells, intracellular acidosis may become significant evens as blood HCO3- decrease.
It is noteworthy, that prior to surgery, my patient's lactate was 7.2 mg/dL, after surgery it had jumped to 12.2 mg/dL. Lactate clearance has been shown to be a much better predictor of mortality than other predictors [2]. In the ARISE trial, septic patients with high lactate levels had higher mortality than those who presented with hypotension only [6]. This massive increase also lends weight to the evidence described above that bicarb therapy may worsen lactate production, although, in this case the patient's on going significant disease burden most likely greatly contributed to the rocketing lactate levels seen. Indeed, 24 hours later, his lactate level had jumped to 18.9 mg/dL and the patient expired not long after this.
Another question that arose early in the case was related to the dose of vasopressin. Due to low blood pressure and the already high dose of norepinephrine, I attempted to increase the infusion rate of vasopressin to 0.05 u/min. The pump had been preprogrammed to disallow this dose. Therefore, I was forced by the pump to limit my infusion to 0.04 u/min. To me this seemed too low of a dose. An excellent review of the pharmacology and endocrinology of vasopressin can be located here. In an early study on vasopressin for septic shock, the dose used to show benefit was 0.07 u/min. In hemorrhagic shock, it is recommended to use doses near 0.4 u/min and in post cardiac surgery vasodilatory shock a dose of 0.1 u/min is recommended and was found to be devoid of adverse effects. Therefore, evidence exists to allow for higher infusion rates of vasopressin when combined with norepinephrine in a patient in severe shock.
Pre-pro-AVP is synthesized in magnocellular neurosecretory neurons (also known as neurohypophyseal neurons) in the supraoptic and paraventricular nuclei (known as osmoreceptors) of the anterior hypothalamus. It migrates along the supraoptic-hypophyseal tract to the posterior pituitary gland, where it is stored in neurosecretory vesicles and is then secreted in response to decreased stretch on atrial, aortic and carotid body mechano receptors. Vasopressin acts on receptors labeled V1, V2 and V3. V1 receptors are located on vascular endothelium, kidneys, platelets, and brain and V2 receptors are predominantly located in the kidney collecting duct where they cause an increase in aquaporin insertion in the walls of the collecting ducts to allow increased water reabsorption into the blood. In refractory shock, the enhanced sensitivity to exogenous vasopressin may be attributable to its ability to block KATP channels, interfere with NO signaling, bind avidly to V1receptors, and potentiate the effects of adrenergic agents at the level of vascular smooth muscle in shock states.
Vasopressin has increased in popularity in ICUs in large part because of large and well done studies such as VASST, where vasopressin (low dose 0.01 to 0.03 mcg/min) was found to be beneficial (decreased mortality by nearly 10%) in patients with low severity shock [4]. Furthermore, in a post hoc analysis of the data from this large study, Gordon found that patients who received vasopressin were less likely to advance to the loss or dialysis categories of RIFLE scoring system if they already had AKI (21% vs 40%) [5]. Unfortunately, in the VASST study, no difference in mortality could be found in those receiving vasopressin in addition to norepinephrine vs norepinephrine alone in patients with more severe sepsis, as was the case in our patient.
The surgery lasted approximately four hours. The patient received seven liters of crystalloid (LR), 1L of albumin and was essentially anuric. Blood loss was minimal. The patient was transported to the ICU ventilated, paralyzed with max pressors continued. The patients lactate nearly doubled within 24 hours, the INR went to 4, d-dimer went very high, Hgb dropped despite no obvious blood loss, liver enzymes went to nearly 5,000, and K+ levels went to 6.9 mEq/L, while albumin dropped to less than 2 mg/dL. The patient was placed on CRRT and it became obvious that he was not going to survive. The patient expired on POD #2 after apparent multi organ failure with DIC.
1. Kim HJ, Son YK, An WS. PLoS One. 2013;8:65283
2. Lee SM, Kim SE, Kim E Bin, Jeong HJ, Son YK, An WS. PLoS One. 2015;10:145181
3. Dünser MW, Mayr AJ, Ulmer H, Knotzer H, Sumann G, Pajk W, Friesenecker B, Hasibeder WR
Circulation. 2003 May 13; 107(18):2313-9.
4. Russell JA, Walley KR, Singer J, Gordon AC, Hébert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM, Cook DJ, Presneill JJ, Ayers D, VASST Investigators.
N Engl J Med. 2008 Feb 28; 358(9):877-87.
5. Gordon AC, Russell JA, Walley KR, Singer J, Ayers D, Storms MM, et al. The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Med. 2010;36:83–91.
6. Gotmaker R, Peake SL, Forbes A, Bellomo R, ARISE Investigators (2017) Mortality is greater in septic patients with hyperlactatemia than with refractory hypotension. Shock 48:294–300
6. Gotmaker R, Peake SL, Forbes A, Bellomo R, ARISE Investigators (2017) Mortality is greater in septic patients with hyperlactatemia than with refractory hypotension. Shock 48:294–300
7.
Zhang
Z
, Zhu
C
, Mo
L
, Hong
Y
: Intensive Care Med
. 2018
; 44
:1888
–95
8.
Jung
B
, Rimmele
T
, Le Goff
C
, Chanques
G
, Corne
P
, Jonquet
O
, Muller
L
, Lefrant
JY
, Guervilly
C
, Papazian
L
, Allaouchiche
B
, Jaber
S
; AzuRea Group
: Crit Care
. 2011
; 15
:R238
9.
Kim
HJ
, Son
YK
, An
WS
: PLoS One
. 2013
; 8
:e65283

No comments:
Post a Comment